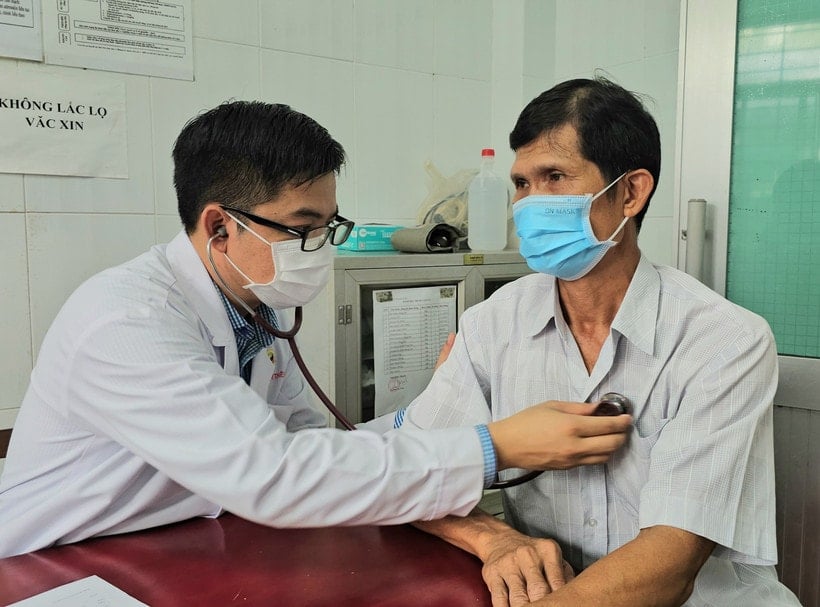
Doctors and nurses give health advice to people
On July 8, according to information from the Ministry of Health , the Ministry issued Circular 26/2025/TT-BYT regulating prescriptions and prescriptions of pharmaceutical and biological drugs in outpatient treatment at medical examination and treatment facilities.
Accordingly, medical examination and treatment facilities organized as hospitals must prescribe drugs electronically before October 1, 2025; Other medical examination and treatment facilities must prescribe drugs electronically before January 1, 2026.
According to Dr. Vuong Anh Duong - Deputy Director of the Department of Medical Examination and Treatment Management (Ministry of Health ), when implementing the provisions of the Circular, the prescription and drug sales systems will be connected. Patients who buy drugs will be controlled according to the prescriptions in the system. Which prescriptions are sold to where, which drugs are sold differently from the prescription, all can be tracked.
This is a huge step forward in controlling the sale of non-prescription drugs, especially antibiotics. The interconnection between the Electronic Prescription System and the National Drug Administration System is one of the key solutions to improve the effectiveness of drug use monitoring.
Circular 26 also requires the integration of personal identification numbers into prescriptions. This is an important step towards synchronizing medical data with the National Population Database System. This is also an implementation in accordance with the spirit of the Government 's Project 06.
When people use their personal identification numbers, many administrative information such as full name, date of birth, gender, address, etc. will be automatically displayed on the system. This will help reduce prescription time, limit errors and simplify procedures for both patients and medical staff.
In addition, Circular 26 also requires prescribers to clearly state the quantity used each time, the number of times used per day, and the number of days the patient uses the medicine.
The Circular also updates new regulations under the 2023 Law on Medical Examination and Treatment (such as prescribing drugs when absolutely necessary, for the right purpose, safely, reasonably and effectively, etc.), and provides clearer instructions on handling addictive drugs, psychotropic drugs, and precursor drugs that have been given to patients but not fully used or the patient has died.
In the coming time, the Ministry of Health will increase technical support, update software, and especially prioritize guidance for grassroots health care - to ensure that all medical examination and treatment facilities can implement effectively.
VN (according to Vietnam+)
Source: https://baohaiphongplus.vn/cac-benh-vien-bat-buoc-phai-ke-don-thuoc-dien-tu-tu-1-10-415872.html
































































































Comment (0)