Illustration of powdered milk.
According to the penalty decision, the enterprise was found to have committed 3 violations.
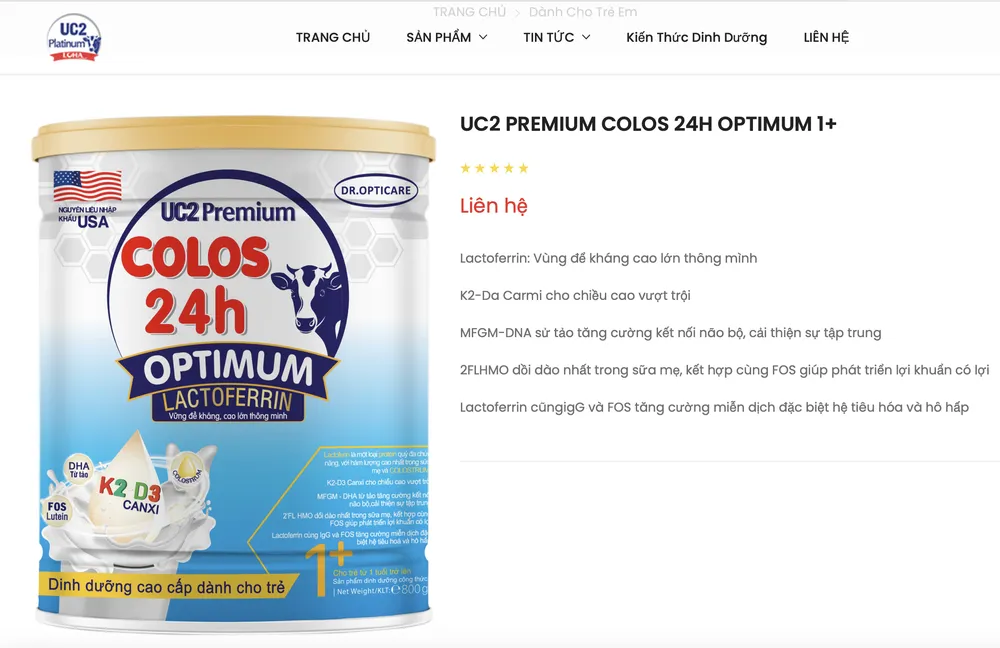
Firstly , the product UC2 Premium Colos 24h Optimum 1+ circulating on the market does not comply with the announced standards. The test results of the Ho Chi Minh City Institute of Public Health showed that the Lysine, Taurin, and DHA indicators did not meet the announced records. The total number of violations was 20 cans, worth more than 3.5 million VND, of which 12 cans were consumed.
Second , the company self-declared two products that must be registered for declaration, including: UC2 Platinum Grow Plus and UC2 Platinum Colos Grow Plus+ , although both clearly state that the target users are children up to 36 months old. This is a group of foods that must be registered instead of self-declared.
Third, the product label violates regulations when it incorrectly shows the place of manufacture. specifically, the product UC2 Premium Colos 24h Optimum 1+ is manufactured at Loha Pharma LLC, but the label states that it is manufactured at Branch 2 - Loha Pharmaceutical Nutrition Import-Export JSC.
UC2 Premium Colos 24h Optimum 1+ product on the company's website.
For the above violations, in addition to being fined 94 million VND, the enterprise was also forced to recall all violating products, including:
- Destroy or change the purpose of use for UC2 Premium Colos 24h Optimum 1+;
- Recall of pre-mixed products UC2 Platinum Grow Plus and UC2 Platinum Colos Grow Plus+;
- Revocation of self-declaration and label of infringing goods;
- Pay back the amount corresponding to the value of the consumed product.
TRANSPORTATION
Source: https://www.sggp.org.vn/ly-do-mot-dong-sua-dinh-duong-cho-tre-em-tai-tphcm-bi-thu-hoi-post802895.html


































































































Comment (0)