The Drug Administration of Vietnam ( Ministry of Health ) has announced the suspension of circulation and nationwide recall of Désembre Derma Science High Frequency Cream Professional (Receipt of Declaration No.: 211274/23/CBMPQLD/23/CBMP-QLD issued on September 2, 2023) by Hyunjin C&T International Co., Ltd. responsible for bringing the product to the market.
Product manufactured by Hyunjin C&T CO.,LTD (Korea).
The Drug Administration of Vietnam said the reason for the recall was that the cosmetics in circulation had formulas and labels with uses that were not consistent with the published records.
The Drug Administration of Vietnam requests the local Department of Health to notify cosmetic businesses and users in the area to immediately stop trading and using the above products and return them to the suppliers; recall violating products, inspect and supervise units implementing this notice; and handle violators according to current regulations.
Along with the decision to recall the product, the Drug Administration of Vietnam also revoked the Cosmetic Product Declaration Receipt Number issued by the Department for this cosmetic. At the same time, it announced a temporary suspension of reviewing and receiving cosmetic product declaration dossiers for HyunJin C&T International Co., Ltd. for a period of 6 months from June 30.
The application for the issuance of a receipt number for the Cosmetic Product Declaration Form submitted by this company before June 30 will no longer be valid. When the suspension period for receiving applications expires, the company wishing to declare its cosmetic products must submit an application according to regulations.
The Drug Administration also requested Hyunjin C&T International Co., Ltd. to send recall notices to distributors and users of the above products; receive returned products from business establishments and recall all products that do not meet regulations; Send product recall reports to the Drug Administration before July 10, 2025.
The Drug Administration of Vietnam requests the Hanoi Department of Health to supervise Hyunjin C&T International Co., Ltd. in recalling the above products that do not meet regulations; report the supervision results to the Drug Administration of Vietnam before July 15, 2025./.
Source: https://www.vietnamplus.vn/thu-hoi-my-pham-co-cong-thuc-nhan-ghi-cong-dung-khong-dung-ho-so-cong-bo-post1047633.vnp














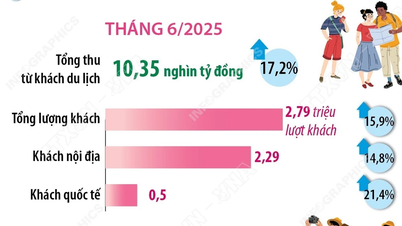






















































































Comment (0)