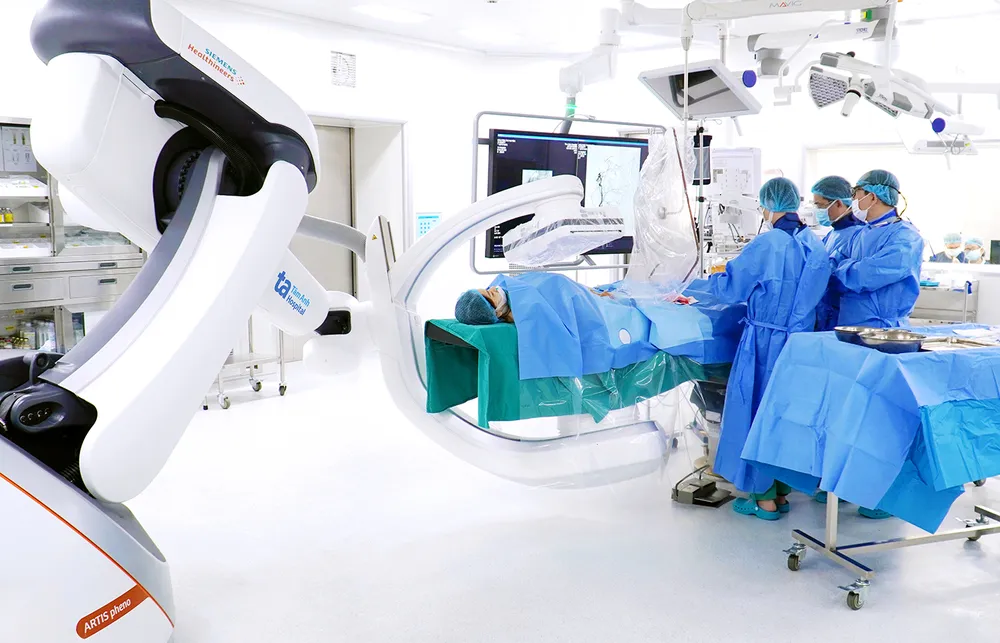
Reduce costs, increase treatment opportunities
At the end of December 2024, Tam Anh Research Institute implemented the VISTA-1 project on oral immunotherapy drugs for cancer treatment in Vietnam. After nearly 6 months of testing, 8 patients with late-stage colorectal cancer were treated according to the research protocol, with no significant side effects related to the research drug and positive signals initially recorded.
As the first person to test the drug in this project, Mr. M. (50 years old), who has colorectal cancer that has metastasized to the liver, said that he had undergone different treatment regimens such as chemotherapy and targeted drugs, but did not respond. In January 2025, he registered to participate in the study and was assessed as eligible to participate. "I used to think that I had no other choice, but thanks to the opportunity to participate in this study, I feel relieved and have more hope," Mr. M. shared.
According to Dr. Vu Huu Khiem, Head of the Oncology Department, Tam Anh General Hospital, Hanoi , patients participating in the study are closely monitored for safety and response to the research drug. The research team has not recorded any unusual symptoms related to the research drug in patients using the drug in the past 3 months.
“For patients with advanced colorectal cancer who no longer respond to current treatment regimens, each improvement in survival time during the research process is very meaningful. We and the patients have now entered the fourth month of implementing this research,” said Dr. Vu Huu Khiem.
At the Ho Chi Minh City Oncology Hospital, Dr. Phan Tan Thuan, Head of the Clinical Trial Unit, said that the unit is currently implementing 37 clinical studies, most of which are in cooperation with multinational pharmaceutical companies (Pharma), partly because the hospital also actively coordinates with cancer centers in the region to conduct independent studies that have academic value and practical applications. The hospital often uses new generation drugs to compare with the standard regimen currently being applied. Many of these drugs are not yet available in Vietnam and are very expensive, some cost up to 300 million VND per treatment cycle (from 3-4 weeks). Participating in the study, patients are given free drugs, regardless of which branch they belong to.
“In addition to helping patients access modern treatment sooner, these studies also create conditions for domestic experts to master data and evaluate drug effectiveness on the Vietnamese population. From there, shorten the time to bring new drugs to the market. Many new generation drugs are very expensive. Here, we give free access to new generation drugs to patients participating in the study. When the research drug is successful, it can shorten the time to bring new drugs to the market,” informed Dr. Phan Tan Thuan.
"Clinical trials over the past time have helped the medical industry quickly access modern treatments, from traditional chemotherapy to immunotherapy and targeted drugs. These advances not only help prolong life but also improve the quality of life for patients, while expanding access to new drugs at lower costs for patients and the medical system."
Deputy Minister of Health NGUYEN THI LIEN HUONG
Lots of potential, lack of mechanism
According to Professor Guy Thwaites, Director of the Oxford University Clinical Research Unit, Vietnam has been at the forefront of antimalarial drug trials since 1991, benefiting millions of patients globally. With a population of about 100 million, Vietnam provides a diverse patient group, ideal for clinical trials. The average GDP growth rate of 6%-7% per year and a growing middle class further increase Vietnam's attractiveness to domestic and foreign sponsors. Despite its great potential, the clinical trial market in Vietnam is still facing many barriers that make development slow and unsynchronized. The approval time for a clinical trial in Vietnam can be up to 160 days - the longest in Asia, while the average time for other countries is about 75 days. In fact, Vietnam is far behind many countries in the region such as Singapore (18 days), Indonesia (20 days), Japan (31 days)... This timeline affects the attractiveness of the Vietnamese market to research sponsors.
In addition, clinical trials require close coordination between doctors, nurses, research coordinators and data managers. However, Vietnam currently lacks a well-trained team; financial difficulties and incentive mechanisms. A phase 3 trial can cost from 10 to 20 million USD. Meanwhile, many investors have reported that Vietnam lacks tax incentives, research funding or co-funding mechanisms like those in Singapore and South Korea. Funding disbursement is often delayed due to administrative procedures, affecting trial progress and reducing competitiveness compared to other countries in the region.
To develop the clinical trial industry in Vietnam, Dr. Phan Tan Thuan said that it is necessary to simplify the approval procedure through a centralized online system to shorten the current complicated approval process. In addition, it is necessary to use the online registration form through an online portal in English, deploy bilingual documents to streamline the process for international sponsors. To compete with countries in the region, it is necessary to develop preferential policies such as tax exemptions, research funding or co-financing mechanisms for trial costs like the programs in Singapore and Thailand.
“Vietnam should promote the public-private partnership model in the field of clinical trial research, helping to share risks and increase resources for sustainable development of the industry. Along with that, it is necessary to have policies to invest in infrastructure, have more testing facilities, and expand specialized training programs for personnel through cooperation with universities and international organizations,” Dr. Phan Tan Thuan proposed.
Mr. LUKE TRELOAR, Head of Infrastructure, Government and Healthcare, KPMG Vietnam: Building Incentive Policies
The clinical trial approval process in Vietnam involves many steps. First, local approval takes 3-12 months depending on the number and location of studies. Next, the approval process at the Ministry of Health takes an average of 3-5 months. Finally, the application for a drug import license (for drug trials) takes an average of 3-4 months. The number of centers and clinical trials in Vietnam is too low compared to the total population, and there is a risk of falling behind in the race to become a regional clinical research center. Vietnam needs policies to invest in infrastructure, have more testing facilities, and expand specialized training programs for personnel through cooperation with universities and international organizations. To compete with countries in the region, it is necessary to develop preferential policies such as tax exemptions, research funding or co-financing mechanisms for testing costs like the programs in Singapore and Thailand.
Mr. NGUYEN NGO QUANG, Director of the Department of Science, Technology and Training (Ministry of Health): Standardizing clinical trial activities
In the coming time, the Ministry of Health will continue to improve the legal framework, standardize clinical trial activities towards international standards. At the same time, strengthen the capacity of the National Council of Ethics in Biomedical Research. The Ministry of Health is currently seeking comments on the draft Circular detailing clinical drug trial activities. This Circular will detail clinical drug trial activities, including principles, standards, procedures and responsibilities related to drug trials on volunteers to assess the safety and efficacy of drugs, determine adverse drug reactions (ADRs) and study drug pharmacokinetics. According to the draft Circular, the principles of clinical drug trials must ensure scientificity, objectivity, honesty, transparency and protect the rights of research participants.
MINH NAM - MINH KHA NG recorded
Source: https://www.sggp.org.vn/gian-nan-thu-nghiem-lam-sang-post802539.html




![[Photo] Red flag with yellow star flutters in France on National Day September 2](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/f6fc12215220488bb859230b86b9cc12)
![[Photo] Politburo works with the Standing Committee of Cao Bang Provincial Party Committee and Hue City Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/fee8a847b1ff45188749eb0299c512b2)
![[Photo] General Secretary To Lam attends the opening ceremony of the National Achievements Exhibition](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/d371751d37634474bb3d91c6f701be7f)
![[Photo] National Assembly Chairman Tran Thanh Man holds talks with New Zealand Parliament Chairman](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/c90fcbe09a1d4a028b7623ae366b741d)

























































































Comment (0)