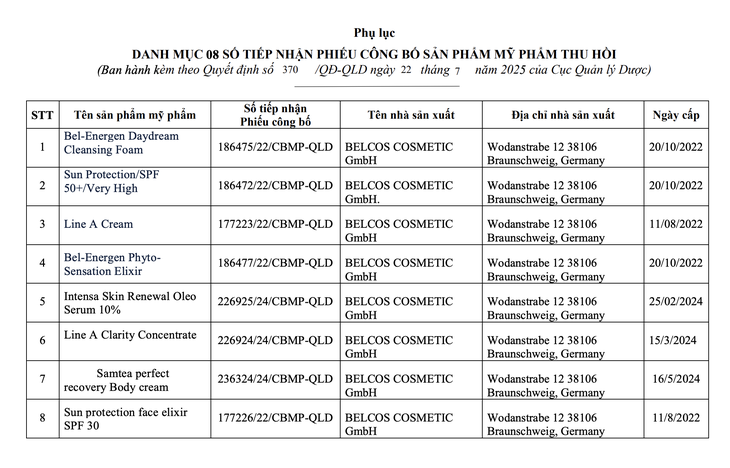
List of products whose product declarations have been revoked - Photo: Department of Drug Administration
The Drug Administration Department decided to withdraw 8 numbers of cosmetic product declaration receipts for cosmetic products of Van Minh Trading Company Limited (No. 10, Lane 28, Phuong Mai Street, Hanoi City) responsible for bringing the product to the market.
Products whose cosmetic declarations have been recalled include: Bel-Energen Daydream Cleansing Foam; Sun Protection/SPF 50+/Very High; Line A Cream; Bel-Energen PhytoSensation Elixir; Intensa Skin Renewal Oleo Serum 10%; Line A Clarity Concentrate; Samtea perfect recovery Body cream; Sun protection face elixir SPF 30. The products are manufactured in Germany, imported and distributed in Vietnam.
The reason for the recall is that the cosmetic products have formulas that do not match the declared documents, the cosmetics in circulation have labels that misrepresent the inherent nature and features of the product.
At the same time, the Department also decided to temporarily stop reviewing and receiving cosmetic product declaration dossiers for Van Minh Company, within 6 months from the date of issuance.
The application for a cosmetic product declaration receipt number submitted by Van Minh Company before July 23 will no longer be valid.
When the suspension period for receiving dossiers expires, companies wishing to declare cosmetic products must submit dossiers according to regulations. The suspension of receiving dossiers of the company is due to the fact that this company sells cosmetics with formulas that are not in accordance with the declaration dossier.
The withdrawal of product declaration forms and the temporary suspension of receiving applications from violating companies show that the Drug Administration is tightening the management of violating cosmetics.
Source: https://tuoitre.vn/thu-hoi-8-phieu-cong-bo-san-pham-my-pham-cua-cong-ty-van-minh-20250723145124454.htm





































































































Comment (0)