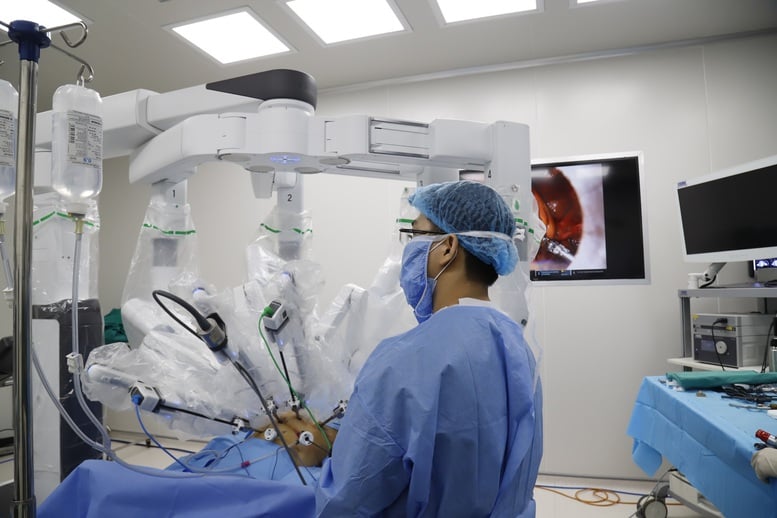
Strictly control the quality of purchased medical equipment, ensure consistency in technology and types, meet professional requirements and ensure patient safety.
According to the Ministry of Health , the practice of purchasing medical equipment and testing supplies in Vietnam in recent times has revealed many shortcomings, requiring more synchronous and effective solutions, specifically:
Currently, the procurement of medical equipment and testing supplies is mainly carried out by each medical facility. This leads to dispersion and lack of uniformity in types, quality and technology among units, causing difficulties in management, maintenance and technology transfer.
Due to small-scale purchasing, medical facilities often face disadvantages in price negotiations with suppliers, leading to higher purchase prices than potential, wasting the state budget and health insurance fund.
The bidding process is complicated, requiring many procedures and human resources, creating a large administrative burden for medical facilities, reducing the effectiveness of professional activities. Distributed procurement and the lack of a centralized control mechanism can create loopholes for negative and opaque behaviors, causing public outrage.
Therefore, the issuance of a Circular on the list of national centralized procurement of medical equipment and testing supplies is necessary to overcome the above limitations, improve efficiency and transparency in procurement, and at the same time ensure the quality of medical examination and treatment and the rights of patients.
The promulgated circular will create a legal corridor to build a national centralized procurement list for medical equipment and testing supplies for unified management, contributing to improving efficiency and professionalism in procurement work of the health sector.
Optimize the use of state budget and health insurance fund through bulk purchasing, enhance product cost reduction. Strictly control the quality of purchased medical equipment, ensure consistency in technology and type, meet professional requirements and patient safety.
Enhance transparency, prevent corruption; standardize processes, publicize information, limit subjective and negative factors in procurement activities. Reduce bidding workload for medical facilities, helping units focus resources on professional medical examination and treatment.
List of national centralized procurement for medical equipment and testing supplies
The draft specifies in detail the names and characteristics of 20 medical devices and testing supplies belonging to 6 groups of medical devices in the national centralized procurement list, including:
1- Artificial lens.
2- Coronary artery stent.
3- Balloon used in cardiovascular intervention.
4- Infusion line.
5- Plastic syringe.
6- Quick test.
Regarding administrative procedures: The Ministry of Health said that the issued Circular will not create administrative procedures but only stipulate a list of national centralized procurement for medical equipment and testing supplies.
Please read the full draft and give your comments here.
Minh Hien
Source: https://baochinhphu.vn/kiem-soat-chat-che-chat-luong-thiet-bi-y-te-duoc-mua-sam-102250722153603372.htm





































































































Comment (0)