Immediately stop selling and using 20 cosmetic products
The Drug Administration of Vietnam ( Ministry of Health ) has just announced the suspension of circulation and nationwide recall of 20 batches of cosmetic products that Dai Cat A International Company Limited (registered address at Ward 9, Phu Nhuan District, Ho Chi Minh City) is responsible for bringing to the market.
Of which, 2 products: Queendoes rhea serum (reception number 241190/24/CBMP-QLD) and Renewing concentrate (reception number 232960/24/CBMP-QLD) were recalled and destroyed due to having formulas that were not as announced in the dossier.
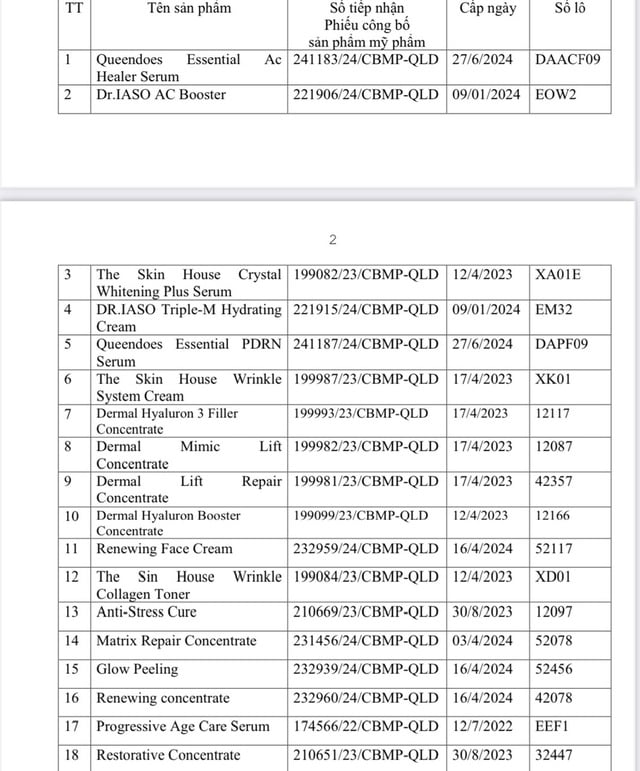
18 cosmetics were recalled due to labels stating uses inconsistent with the published records.
PHOTO: DAV.GOV.VN
18 other cosmetics released by the company were recalled because their labels stated uses inconsistent with the published records.
The Drug Administration requires the destruction of products if the violating elements cannot be removed (the violating label cannot be separated from the goods).
Dai Cat A International Company Limited is required to send recall notices to distributors and users of the 20 above-mentioned products; receive returned products from business establishments and recall all 20 products that do not meet regulations.
The Drug Administration of Vietnam requested the Ho Chi Minh City Department of Health to supervise Dai Cat A International Company Limited in recalling the above 20 products that do not meet regulations.
The Department of Health of provinces and cities shall notify businesses and cosmetic users in the area to immediately stop trading and using the 20 products mentioned above and return them to the suppliers. At the same time, the violating units shall be recalled and handled according to regulations.
Source: https://thanhnien.vn/yeu-cau-thu-hoi-ngung-su-dung-tren-toan-quoc-20-my-pham-185250623184829797.htm



![[Photo] Chu Dau Ceramics – Proud of Vietnamese identity at Exhibition A80](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/1/c62ab2fc69664657b3f03bea2c59c90e)
![[Photo] National Assembly Chairman Tran Thanh Man receives Cambodian Senate President Hun Sen](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/1/7a90c9b1c1484321bbb0fadceef6559b)
![[Photo] People eagerly wait all night for the parade on the morning of September 2](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/1/0cf8423e8a4e454094f0bace35c9a392)
![[Photo] Celebration of the 65th Anniversary of the Establishment of Diplomatic Relations between Vietnam and Cuba](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/1/0ed159f3f19344e497ab652956b15cca)
![[Photo] Solemn reception to celebrate the 80th anniversary of the National Day of the Socialist Republic of Vietnam](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/1/e86d78396477453cbfab255db1e2bdb1)
![[Photo] General Secretary receives heads of political party delegations from countries attending the 80th anniversary of our country's National Day](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/1/ad0cb56026294afcae85480562c2e790)




































































![[Infographic] A week of strong fluctuations, gold prices continuously set historical peaks](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/9/1/1520fa24c9c94c39afee9a58ceb09de1)
























Comment (0)