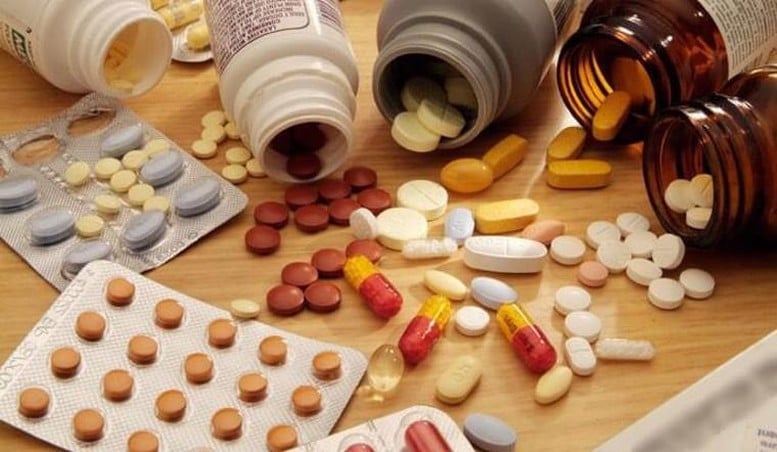
Revoking the Declaration Acceptance Certificate of 17 health protection food products
The Food Safety Department has just issued Decisions to recall a number of health protection foods of Nguyen Minh Pharmaceutical and Trading Company Limited and Vy Vy Vietnam Trading Company Limited, Thanh Truc Production and Trading Facility Business Household, USA VIP Pharmaceutical Group Company Limited, Dang Thien Phuc Business Household, and Dang An Khang Business Household.
Accordingly, in Decision No. 321/QD-ATTP , the Food Safety Department revoked the validity of 2 Certificates of registration of health protection food product declaration : Hoa Bao Weight Gain Pills, Certificate No. 283/2018/DKSP, issued on March 20, 2018; Hoan Lieu Khang, Certificate No. 2694/2018/DKSP dated May 21, 2018. These health protection food products were announced by Vy Vy Vietnam Trading Company Limited (Thai Nguyen), now BAO AN CONSTRUCTION - INTERIOR TRADING COMPANY LIMITED ( Hanoi City).
In Decision No. 323/QD-ATTP , the Department of Food Safety revoked the validity of the Product Declaration Registration Receipt No. 5803/2019/DKSP dated May 23, 2019 of the health protection food product ENTEROVINA announced by Nguyen Minh Pharmaceutical and Trading Company Limited (Hanoi City).
In Decision No. 328/QD-ATTP , the Department of Food Safety revoked the validity of 4 Certificates of registration of the declaration of health protection food products Thoai Cot Hoan with Certificate No. 425/2019/DKSP dated January 9, 2019; Cot Thong Ky Sanh Hoan with Certificate No. 5685/2019/DKSP dated May 22, 2019; Vien Khop Hoan with Certificate No. 2113/2023/DKSP; TyVieXoan with Certificate No. 6180/2019/DKSP dated June 3, 2019. These health protection food products were announced by Dang Thien Phuc Business Household ( An Giang ).
In Decision No. 327/QD-ATTP , the Food Safety Department revoked the validity of 3 Certificates of registration of declaration of health protection food products Eucavital plus Certificate of receipt No. 5617/2023/DKSP dated July 2, 2023; ZN+VITC SYRUP Certificate of receipt No. 2692/2022/DKSP dated April 25, 2022; Vitamin 3B Plus Gold Certificate of receipt No. 10186/2023/DKSP dated November 3, 2021. These health protection food products were announced by USA VIP Pharmaceutical Group Co., Ltd. ( Ho Chi Minh City).
In Decision No. 326/QD-ATTP, the Food Safety Department revoked the validity of 2 Certificates of registration of the declaration of health protection food products Bach Minh Vuong Certificate of Acceptance No. 1460/2023/DKSP dated February 14, 2023; Vixoa Bach Minh Certificate of Acceptance No. 2617/2023/DKSP dated March 16, 2023. These health protection food products were announced by Thanh Truc Production and Trading Business Household (An Giang).
In Decision No. 329/QD-ATTP, the Food Safety Department revoked the validity of 5 Certificates of registration of declaration of health protection food products Cot Thong Hoan, Certificate No. 5356/2022/DKSP dated September 28, 2022; Ty Viem Hoan, Certificate No. 7747/2019/DKSP dated June 24, 2019; An Khop Hoan, Certificate No. 487/2023/DKSP dated January 12, 2023; Rheumatoid Arthritis Cot Thong Hoan, Certificate No. 8516/2018/DKSP dated October 24, 2018; Vung Cot Hoan, Certificate No. 5150/2022/DKSP dated September 13, 2022. These health protection food products were declared by Dang An Khang Business Household (An Giang).
According to the Food Safety Department, the Department's decision was based on the company's request to withdraw the dossier to declare all of these health food products. The Department's decisions are effective from July 2, 2025.
TB
Source: https://baochinhphu.vn/thu-hoi-giay-tiep-nhan-cong-bo-cua-17-san-pham-thuc-pham-bao-ve-suc-khoe-102250714153524677.htm



![[Photo] Red flag with yellow star flutters in France on National Day September 2](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/f6fc12215220488bb859230b86b9cc12)
![[Photo] Politburo works with the Standing Committee of Cao Bang Provincial Party Committee and Hue City Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/fee8a847b1ff45188749eb0299c512b2)
![[Photo] General Secretary To Lam presents the 45-year Party membership badge to comrade Phan Dinh Trac](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/e2f08c400e504e38ac694bc6142ac331)
![[Photo] Prime Minister Pham Minh Chinh meets with Speaker of the New Zealand Parliament Gerry Brownlee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/cec2630220ec49efbb04030e664995db)
































































































Comment (0)