8 food products businesses request to withdraw application
The Food Safety Department ( Ministry of Health ) has just decided to revoke the validity of the certificate of registration of the product declaration of health protection food Kingphar super kids (certificate of registration of product declaration number 13714/2019/DKSP). The product was announced by Vinh Thinh Vuong Production and Trading Company Limited (located in Hung Yen province) and has an official dispatch requesting to withdraw the dossier related to the product.
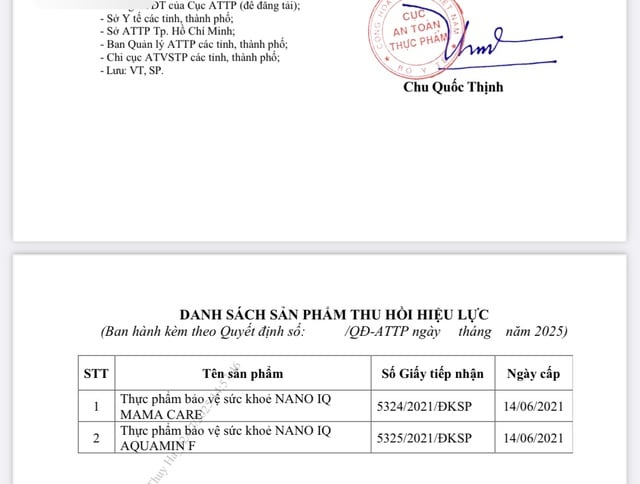
Products with a decision by the Food Safety Department to revoke the product declaration registration certificate
PHOTO: VFA.GOV.VN
The Food Safety Department also revoked the validity of the product declaration registration certificate for the health protection food product Enterovina (product declaration registration certificate number 5803/2019/DKSP). The product was announced by Nguyen Minh Trading and Pharmaceutical Company Limited ( Hanoi ) and requested to withdraw the product-related documents.
4 health food products: Nato thong huyet Kingphar (6074/2020/DKSP); Menmoren Ginkgo Plus Q10 (6303/2020/DKSP); Digestive enzyme tube (10160/2020/DKSP); Omega 3-6-9 Plus Q10 Kingphar (3068/2021/DKSP), also had a decision to revoke the validity of the product registration certificate, issued by the Department of Food Safety. 4 products were announced by Kingphar Vietnam Joint Stock Company ( Hung Yen ) and requested to withdraw the product profile.
In addition, the Food Safety Department has decided to revoke the validity of the product registration certificate for 2 health protection foods: Nano IQ mama care (5324/2021/DKSP) and Nano IQ aquamin F (5325/2021/DKSP). The 2 products were announced by Nano Pharma Pharmaceutical Joint Stock Company (Hanoi) and requested to withdraw the product profile.
The above products have introductory information: providing vitamins to help children eat well, strengthen immunity; prevent digestive disorders; increase blood circulation, dissolve blood clots; products for pregnant women...
Previously, in June, the Food Safety Department revoked the validity of product declaration registration certificates for many health food products.
Source: https://thanhnien.vn/thu-hoi-cong-bo-8-san-pham-bao-ve-suc-khoe-185250703114956898.htm




![[Photo] General Secretary To Lam attends the opening ceremony of the National Achievements Exhibition](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/d371751d37634474bb3d91c6f701be7f)
![[Photo] Politburo works with the Standing Committee of Cao Bang Provincial Party Committee and Hue City Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/fee8a847b1ff45188749eb0299c512b2)
![[Photo] Red flag with yellow star flutters in France on National Day September 2](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/f6fc12215220488bb859230b86b9cc12)
![[Photo] General Secretary To Lam presents the 45-year Party membership badge to comrade Phan Dinh Trac](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/e2f08c400e504e38ac694bc6142ac331)
![[Photo] Prime Minister Pham Minh Chinh meets with Speaker of the New Zealand Parliament Gerry Brownlee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/28/cec2630220ec49efbb04030e664995db)


























































































Comment (0)