The Drug Administration of Vietnam, Ministry of Health, informed that among more than 500 drugs and generic drugs newly granted and having their circulation registration numbers extended this time, there are 414 domestically produced drugs and 68 generic drugs...
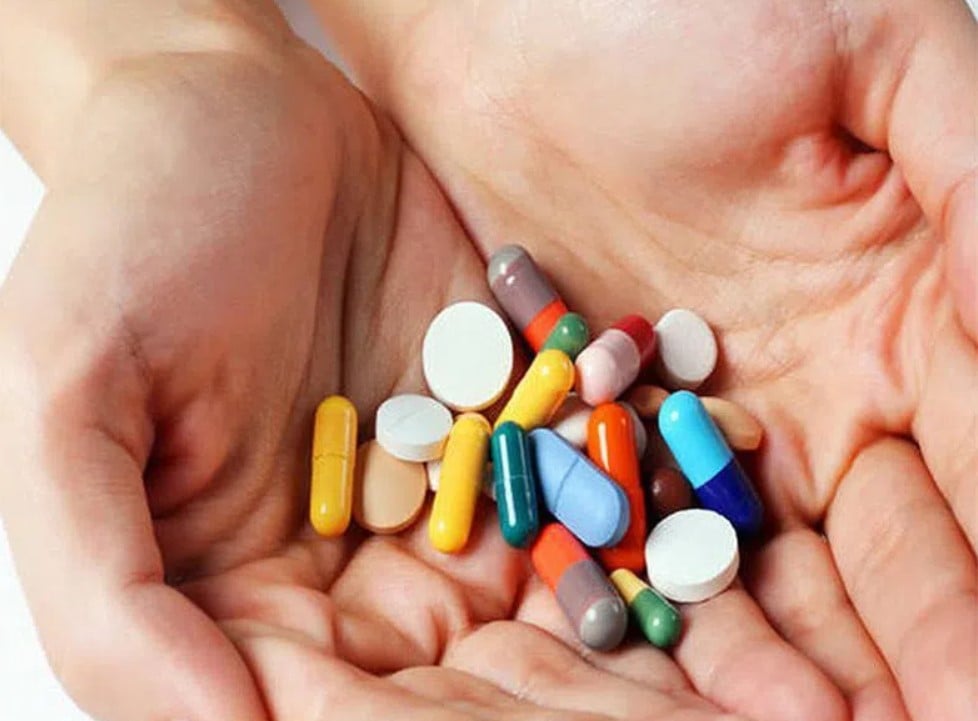
500 original drugs, medicines... have just been newly granted and have their registration numbers renewed by the Ministry of Health .
Previously, in March and early April 2024, the Ministry of Health issued new, extended and announced thousands of domestically produced drugs, generic drugs and drugs and pharmaceutical ingredients to implement Resolution 80 of the National Assembly (12th extension announcement).
It is known that the original pharmaceutical products and brand-name drugs that have been newly granted circulation registration numbers and have had their registration numbers extended in the past are quite diverse in terms of pharmacological effects, including drugs for treating cancer, cardiovascular disease, hypertension, diabetes, antiviral drugs, drugs for treating respiratory diseases, antibiotics, antipyretics, analgesics, other common anti-inflammatory drugs... and vaccines and biological products that are in high demand for use in medical examination and treatment and disease prevention.
The Ministry of Health requires that drug manufacturing and registration establishments are responsible for manufacturing drugs in accordance with the records and documents registered with the Ministry of Health and must print the registration number issued by the Vietnamese Ministry of Health on the drug label...
The Ministry of Health also requires drug manufacturing and registration facilities to coordinate with treatment facilities to comply with current regulations on prescription drugs, monitor the safety, effectiveness, and adverse effects of drugs on Vietnamese people, and synthesize and report according to regulations.
The drug registration facility must ensure that the operating conditions are maintained during the validity period of the drug and drug ingredient registration certificate. In case the operating conditions are no longer met, the registration facility must be responsible for changing the registration facility according to regulations.
Drug manufacturing facilities must ensure the operating conditions of the manufacturing facility during the validity period of the drug and drug ingredient circulation registration certificate.
Source: https://www.baogiaothong.vn/bo-y-te-cap-moi-gia-han-hon-500-thuoc-biet-duoc-192240513105520481.htm























![[Photo] President Luong Cuong's wife and Queen of Bhutan visit Tran Quoc Pagoda](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/19/62696af3852a44c8823ec52b03c3beb0)
![[Photo] General Secretary To Lam attends the inauguration and groundbreaking ceremony of 250 projects to celebrate National Day](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/19/3aa7478438a8470e9c63f4951a16248b)


![[Photo] General Secretary and Prime Minister visit the National Exhibition and Fair Center](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/19/f4503ad032d24a90beb39eb71c2a583f)
![[Photo] Close-up of the first International Financial Center building in Ho Chi Minh City](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/19/3f06082e1b534742a13b7029b76c69b6)


![[Photo] Politburo works with the Standing Committee of Da Nang City Party Committee and Quang Ninh Provincial Party Committee](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/19/b1678391898c4d32a05132bec02dd6e1)

































































Comment (0)