On June 23, according to information from the Ministry of Health , the Drug Administration of Vietnam sent a document to the Department of Health of provinces and centrally-run cities and Cuu Long Pharmaceutical Joint Stock Company requesting the recall of the company's drug batch due to poor quality.
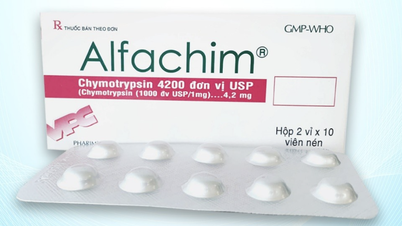
The recalled batch of medicine is labeled Alfachim 4.2 tablets (Chymotrypsin (equivalent to 21 microkatal chymotrypsin) 4200 IU), Registration number: VD-34573-20, Batch number 03010624; Manufacturing date: June 1, 2024; Expiry date: June 1, 2026, manufactured by Cuu Long Pharmaceutical Joint Stock Company.
Previously, in May 2025, the Central Institute for Drug Control tested the above drug sample, the result did not meet the quality standards for quantitative indicators (level 2 violation). The Drug Administration requested to recall this batch of drugs in Hanoi ; at the same time, requested Cuu Long Pharmaceutical Joint Stock Company to coordinate in taking additional samples and sending the samples to the Central Institute for Drug Control or Ho Chi Minh City Institute for Drug Control to test the quality for quantitative indicators.
However, after the 15-day deadline as requested, Cuu Long Pharmaceutical Joint Stock Company has not yet reported the results of the additional sample quality inspection, only reporting on the production, distribution of the drug and the recall report. The company then sent a document to the Drug Administration, requesting a voluntary recall of this batch of drugs.
Therefore, on June 22, the Drug Administration of Vietnam announced a nationwide recall of this batch of drugs and requested Cuu Long Pharmaceutical Joint Stock Company to coordinate with the drug distributor to quickly recall the entire batch of drugs that do not meet the above quality standards; send a recall report to the Department before July 8, 2025.
The Drug Administration also requested the Department of Health of provinces and centrally-run cities and health sectors to notify drug businesses and users to recall the above-mentioned substandard drug batches, publish information about the drug recall decision on the Department's website, inspect and supervise units implementing this notice; handle violators according to current regulations; report to the Drug Administration and relevant authorities.
The Hanoi Department of Health and the Vinh Long Department of Health will inspect and supervise the company in recalling and handling the above medicine, and at the same time evaluate the effectiveness of the drug recall, whether the product is still able to continue to be circulated and used and has the risk of adversely affecting the health of users.
Alfachim 4.2 tablets of Cuu Long Pharmaceutical Joint Stock Company are anti-edema and anti-inflammatory drugs, used in the treatment of edema after trauma, burns, and after surgery./.
Source: https://www.vietnamplus.vn/bo-y-te-thu-hoi-thuoc-alfachim-42-do-khong-dat-tieu-chuan-post1045786.vnp





































































































Comment (0)