The decision, announced by the US Food and Drug Administration (FDA) on June 19, is a major step forward in the decades-long fight to control the global pandemic, opening up the opportunity to protect millions of people, although access remains a big question mark.
“This could really end HIV transmission,” said Greg Millett, director of public policy at the Foundation for AIDS Research (amfAR).
Unlike traditional vaccines, lenacapavir is an antiviral drug that works by keeping levels of the drug high enough in the body to kill any virus that enters it. It was 96% effective in protecting heterosexual women and 100% effective in protecting men who have sex with men and gender-diverse people, according to two studies by Gilead Sciences, a company that makes the drug. That’s a better result than PrEP pills taken daily.
Lenacapavir opens a new avenue for HIV prevention, a turning point for the global health sector, which has been struggling with declining investment in HIV programs. Dr. David Ho, a pioneer in HIV treatment at Columbia University, called it a "major breakthrough."
Previously, lenacapavir was approved by the FDA in 2022 for the treatment of HIV in drug-resistant patients. In extensive testing, scientists discovered that the drug has two outstanding properties: it lasts long in the body and effectively interferes with the virus' replication process. This is the premise for Gilead to shift the drug's development direction from treatment to prevention.
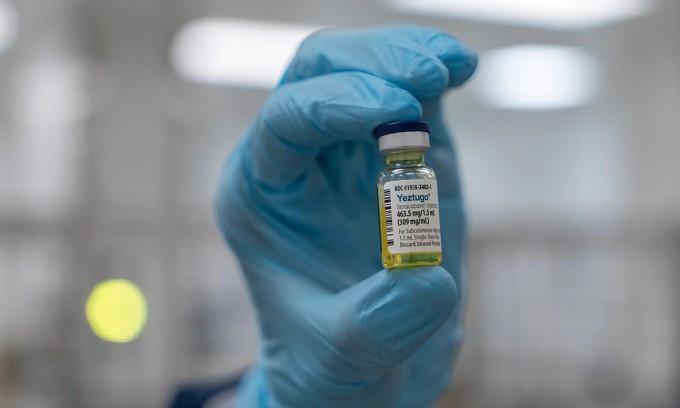
HIV vaccines have yet to show clear efficacy after more than 40 years of research. Lenacapavir, on the other hand, has shown strong protection. This makes it difficult for scientists to design clinical trials for new vaccines, because they cannot ask participants to give up a proven protective option.
The scientific potential of lenacapavir is also threatened by practical hurdles. Patients need to visit a health facility to receive the injection, and they must be tested for HIV before each injection. And cost remains a major barrier. Although Gilead has pledged to make lenacapavir available at a price comparable to current PrEP drugs, the price could still be out of reach for millions of people in low-income countries.
Budget cuts to global health programs like USAID and PEPFAR are threatening the infrastructure for drug distribution. “We just built the best plane in the world , but unfortunately we destroyed all the runways,” warned Kevin Frost, amfAR’s CEO.
To address this, Gilead has licensed the drug to six companies in 120 developing countries royalty-free. Meanwhile, experts are calling for research into a self-injectable form of lenacapavir, like insulin, to overcome the medical barriers in remote areas.
"Long-acting drugs like lenacapavir can help increase adherence and expand access. This is an opportunity to accelerate HIV prevention in the next decade," said Hui Yang, director of the Global Fund to Fight HIV, Tuberculosis and Malaria.
Source: https://baohatinh.vn/my-phe-duyet-thuoc-ngua-hiv-dau-tien-tren-the-gioi-post290186.html





































































































Comment (0)