The newly announced conclusion of the Government Inspectorate shows that during the inspection period, there was a delay in processing applications for granting and renewing registration numbers for drugs and medical equipment... at the Ministry of Health, which is one of the reasons for the shortage of drugs and equipment.
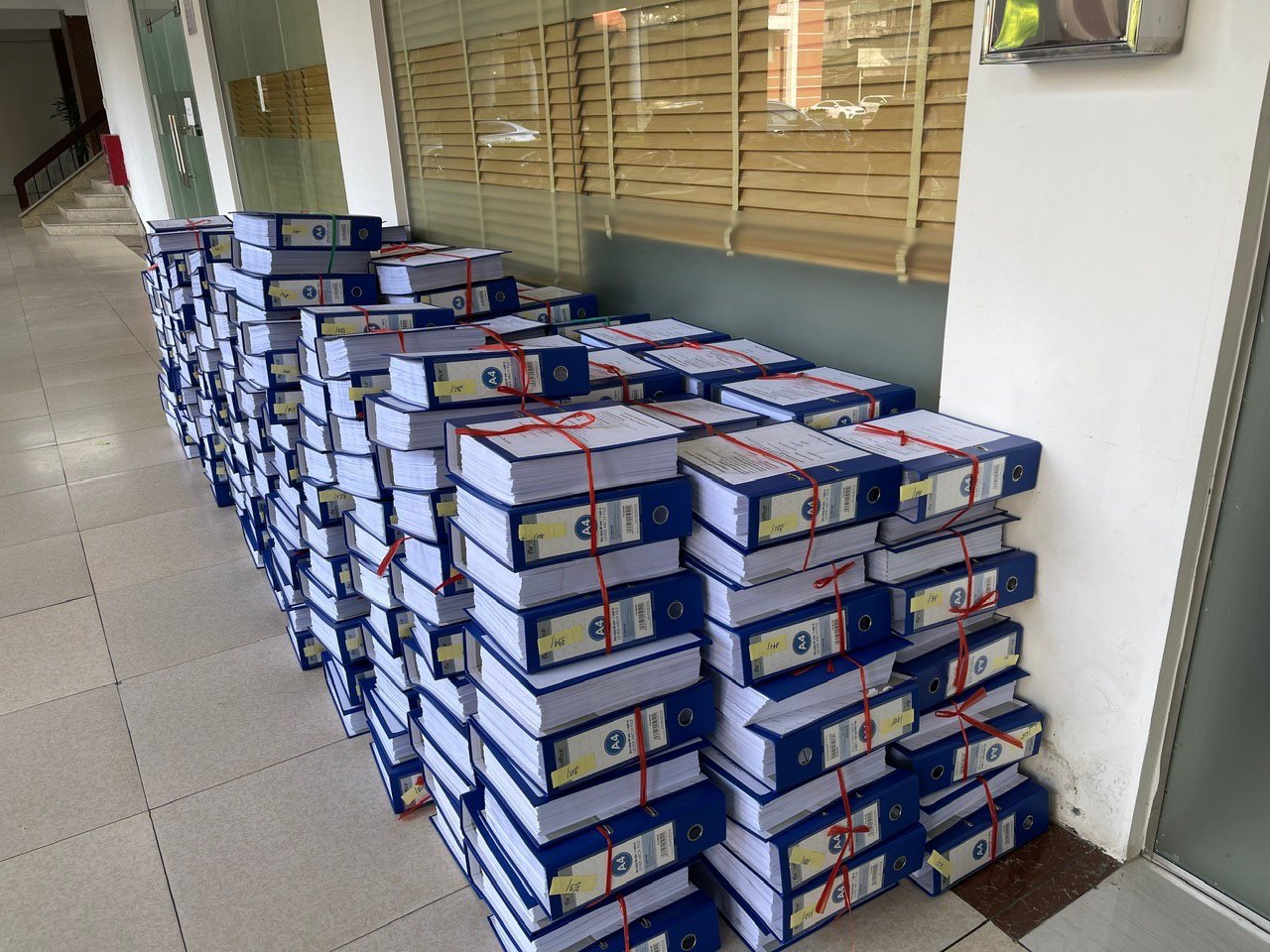
Paper documents for registering a drug submitted to the Drug Administration of Vietnam. According to the agency, the delay in implementing information technology in receiving, monitoring, and notifying the issuance of drug registration documents is a major shortcoming in the period before 2022 - Photo: BYT
This is a matter of concern because recently hospitals have been lacking many medicines and medical equipment. Patients have to wait and spend money on buying medicines and medical supplies outside, even though those medicines and supplies are covered by health insurance.
On December 11, Director of the Drug Administration of Vietnam Vu Tuan Cuong and Deputy Director Nguyen Thanh Lam met with the press to inform about the problems and solutions. Mr. Lam said:
There are many subjective and objective reasons leading to the drug shortage. The market currently has more than 24,000 drug registration numbers, of which in 2024 alone, there were more than 13,000 new and renewed registration numbers. This is also the time when we focus on building the revised Pharmacy Law (amending nearly 50 articles). The inspection results are related to an entire period, including the period 2019 - 2022.
At that time, the mistakes in the VN Pharma case affected the psychology of many people. Up to 36 members of the expert group stopped participating in the appraisal of drug registration dossiers. Four subcommittees did not have experts to read the dossiers, leading to a large backlog. At the same time, up to 36 civil servants of the department quit their jobs, and then the COVID-19 epidemic led to difficulties in the appraisal of dossiers.
This, combined with the reason that more than 20,000 drug registration numbers are more than 20,000 sets of documents, not to mention additional documents, are all paper copies. Every time we send documents to experts to read, we give the courier a lot of document clips, some drug documents have up to 149 paper document files. In the inspection conclusion, the Department of Drug Administration is required to have a solution to immediately overcome the lax management, in this case, the management and monitoring of documents. The lack of effective implementation of information technology at this stage is a major shortcoming leading to insufficient conditions to monitor documents, but not a laxity of state management.

Mr. Nguyen Van Loi, Head of Drug Registration Department, Department of Drug Administration, introduces the current system for receiving and tracking drug registration dossiers - Photo: THUY ANH
Viettimes : How do you explain the situation where businesses have to supplement their documents many times, some businesses supplement 6-7 times, or receive many requests to supplement the same content?
The current drug registration dossier is implemented by 10 ASEAN countries. Regarding the rate of dossiers requiring additional documents, we have reported to the Government that 94% of the dossiers submitted for the first time require additional documents because the quality of the dossier is not up to standard. However, it is not the department that requests it, but the experts who request it. There are also cases where the dossier must be requested for additional documents 6-7 times, but according to the revised Pharmacy Law that will be implemented soon, businesses are only allowed to supplement documents 3 times, which is also a regulation that helps improve the quality of submitted documents.
Regarding the fact that businesses receive many requests to supplement similar content, it is due to the overlap between two documents related to drug prices. When the department receives the drug price declaration, businesses are allowed to sell drugs. This additional request is an administrative request but does not affect the business's sales. The department has explained this issue.
Tuoi Tre: Regarding the processing of drug registration dossiers, some businesses said that recently this has been done faster, but they need to keep track of where the dossiers are and how far they are being processed? The "one-stop" dossier submission system has also been complained about being difficult to log in to...
When it first went into full operation (the system will be fully operational from July 2023), there were some problems for a while, but now it is running smoothly. Businesses can send all their files through the system, and when the files reach the stages and the progress of the processing, the department and the business can both track it, but some information the business will not be able to see, such as which expert is reading this file.
Which part of the department is slow, how slow it is, are clearly displayed and can be monitored, urged, and responsibilities handled...
Viettimes: What is the solution to the existing errors as you mentioned?
The Drug Administration Department has deployed the information technology system for drug registration twice, but it had problems and had to be redone from scratch. With the new system, we spent a lot of time organizing and now businesses do not have to submit paper documents at all, and council meetings are also held online.
Regarding the review of documents, the registration number advisory board used to hold meetings every 2 months, but from 2020 to now, there have been an average of 30 meetings/year, and from 2023 to 2024, there have been 44 meetings/year. Previously, independent experts were invited from 2 medical universities, but now they are invited from 6 universities with 600 experts. The drug registration team has 25 more staff members at the same time as the legal system is completed.
From these changes, the number of drug registrations and renewals granted increased over the years: 1,341 drugs in 2021, 2,721 drugs in 2022, 4,592 drugs in 2023, and 13,164 drugs in 11 months of 2024, equivalent to the number granted in the previous 5 years combined.
Tuoi Tre: As you said, there are many medicines, but many hospitals lack medicines. What is the reason for this?
We surveyed and found that hospitals such as the National Children's Hospital, Hue Central Hospital, Cho Ray Hospital... do not lack medicine, but why are some hospitals lacking? There is a situation of slow purchasing. If pharmacies have it but the hospital does not, it is because the hospital is slow in bidding.
Speaking to Tuoi Tre on the morning of December 11, a representative of a company specializing in importing drugs said that compared to previous years, the issuance of drug registration numbers at the Drug Administration Department is now faster, which is what businesses expect to ensure drug supply and production and business activities.
However, this representative suggested that during the drug registration process, businesses need to know where the dossier is, the status of the settlement, and the department should respond promptly on whether the dossier is qualified or not, what needs to be supplemented in a quick and concise manner, without "soaking" the dossier for years as the Government Inspectorate pointed out.
Source: https://tuoitre.vn/cuc-quan-ly-duoc-noi-gi-ve-cham-tre-cap-gia-han-so-dang-ky-dan-den-thieu-thuoc-20241211182121534.htm





![[INFOGRAPHIC] Sony RX1R III returns: Compact, Full-frame quality](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/8/5/24845e53f6f643f19a203e2e53d69139)
















































![[Maritime News] Two Evergreen ships in a row: More than 50 containers fell into the sea](https://vphoto.vietnam.vn/thumb/402x226/vietnam/resource/IMAGE/2025/8/4/7c4aab5ced9d4b0e893092ffc2be8327)













































Comment (0)