After preclinical trials of this new anti-cancer vaccine were successful in both safety and high efficacy.
The vaccine has undergone preclinical studies according to international standards, lasting for 3 consecutive years. The results showed that it is absolutely safe, even when injected repeatedly, and is highly effective in inhibiting tumor growth.
The results were astonishing, with tumor size and growth rates reduced by 60-80%. Remarkably, the vaccine also had the potential to significantly improve patient survival rates.
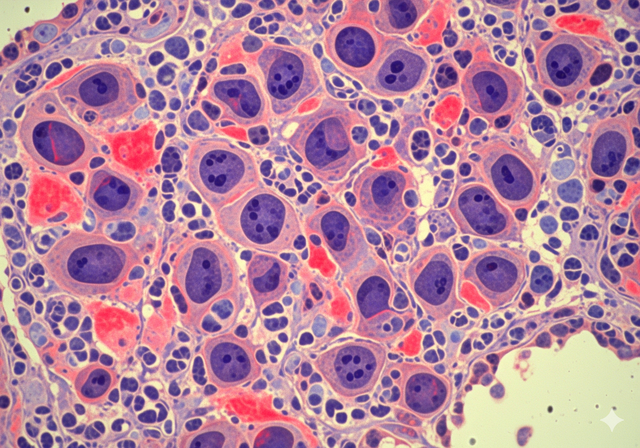
The highlight of this vaccine is its ability to directly affect the immune system, helping the body recognize and fight cancer cells.
Illustration: AI
Pending clinical approval
In late summer 2025, FMBA submitted a complete application to the Russian Ministry of Health for approval of the vaccine for human use. This means that in a very short time, if approved, the vaccine could begin testing in hospitals and oncology centers, according to TASS news agency.
The first target is colorectal cancer.
In the first phase, the vaccine is being targeted at colorectal cancer - one of the most common cancers in the world. In parallel, scientists are also perfecting the development of vaccines against glioblastoma - one of the most malignant tumors, and special types of melanoma.
According to Ms. Skvortsova, the highlight of this vaccine is its ability to directly affect the immune system, helping the body recognize and fight cancer cells. This is a new direction compared to traditional methods such as chemotherapy or radiotherapy.
The vaccine is ready for use, we are just waiting for the license, Skvortsova said. If it is implemented in clinical practice, it could become one of the most important breakthroughs in Russian medicine in cancer treatment.
Source: https://thanhnien.vn/nga-vac-xin-ung-thu-giam-80-khoi-u-da-san-sang-su-dung-185250907160008105.htm




![[Photo] Politburo works with the Standing Committees of Vinh Long and Thai Nguyen Provincial Party Committees](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/8/4f046c454726499e830b662497ea1893)
![[Photo] Politburo works with the Standing Committees of Dong Thap and Quang Tri Provincial Party Committees](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/8/3e1c690a190746faa2d4651ac6ddd01a)






























![[Photo] Amazing total lunar eclipse in many places around the world](https://vphoto.vietnam.vn/thumb/1200x675/vietnam/resource/IMAGE/2025/9/8/7f695f794f1849639ff82b64909a6e3d)
































































Comment (0)